The Iodine Clock Reaction
This is a fascinating reaction which exploits the fact that iodine forms a black complex with starch, but that an even faster reaction takes place simultaneously which prevents this complex from being produced, by ionising the iodine and taking away its ability to react with starch. It is only after the component preventing the starch-complex reaction is totally used up, that the complex is produced and the solution changes colour.
The chemical compounds that we use in the solutions are:
Solution 1 - Hydrogen Peroxide (aqueous) Acidified solution(Usually ethanoic acid)
Solution 2 - Starch, Potassium Iodide and Sodium Thiosulphate
When the solutions are mixed, they are both initially colourless (although the Sodium Thiosulphate solution may be slightly cloudy) and the stop clock is started.
The basic reaction is:
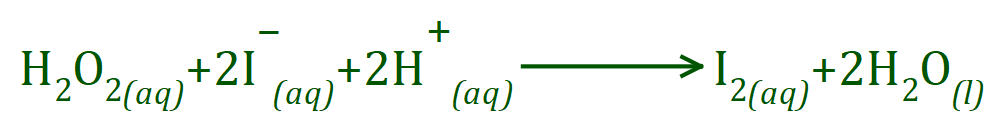
[For more advanced discussions or investigations - this reaction is the rate determining step and is first order with respect to both H2O2 and I–.]
As soon as the iodine is formed, it reacts with the Thiosulfate to form Tetrathionate ions and recycles the iodide ions by the fast reaction:

As soon as all the Thiosulphate is used up, free iodine (or, strictly, I3- Triiodide ions) remains in solution and reacts with the starch to form the familiar blue-black complex.



The time for the blue colour to appear can be adjusted by varying the amount of Thiosulphate in solution so a ‘clock’ of any desired time interval can be produced. To discuss what is happening it is probably easier to think of it like this.
The acidified Hydrogen Peroxide solution oxidises the Iodide ions in solution to elemental Iodine, 2 atoms of which join together to form an Iodine molecule in aqueous solution.
At this point, iodine would normally react with Starch to form the familiar blue/black Starch-Iodine complex, however a much faster reaction is waiting in the wings, in as much as the Thiosulphate ions in solution will pounce on the Iodine molecules as soon as they are formed, reducing the individual atoms back to Iodide ions. They can no longer react with starch in this way, but the Thiosulphate ion pays a price by doing this as it has donated an electron to make this possible, and 2 now electron deficient Thiosulphate ions join together to form a Tetrathionate ion.
As you can probably gather from this, the concentration of Thiosulphate is now starting to reduce, and when it gets to the point where there is no Thiosulphate left, there will be no way to reduce the Iodine back to Iodide, as a result of which it will then react unimpeded with the starch in solution forming the blue-black complex.
This is perhaps one of the best reactions I can think of to give an idea of just exactly what is happening at the molecular level, and just how fast chemical reactions take place in solution.