Gamma Decay
Gamma rays are very short wavelength electromagnetic radiation waves. They have no mass and no electrical charge, being produced by the decaying nuclei of certain elements. Because of their small size and high penetrative power, they are weakly ionising. Gamma rays require thick lead sheeting or extremely thick concrete to stop them.
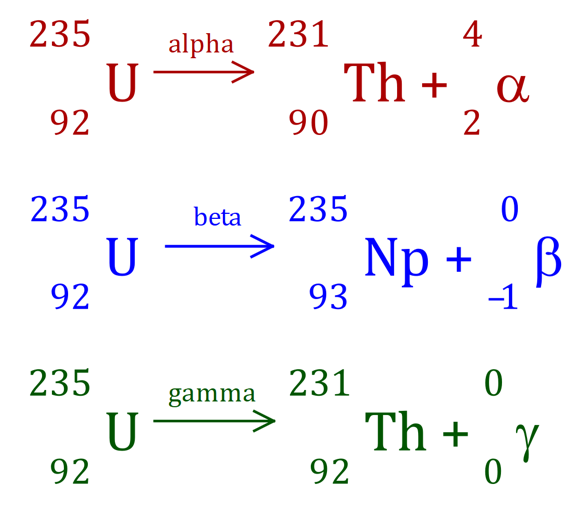
I'm not going to spend very much time in this section because it can get horrendously complicated. If you look at the diagram above you can see that Uranium 235 decays to Thorium 231 by alpha emission.
Similarly Uranium 235 decays by beta emission to Neptunium 235.
Uranium can also decay by gamma emission. When the intermediate "daughter" nucleus is left in an excited state, it returns to the ground state releasing the excess energy as a gamma ray. As I mentioned previously, gamma rays have no mass and no charge.
>> Questions <<
Go To >> Radioactive Decay Pathways <<