Rates of Reaction
Q1. A student reacts Magnesium with five different concentrations of Hydrochloric Acid, as shown in the data table. The time (in seconds) for Hydrogen gas to stop being produced is also shown.
(a) On the same graph plot both sets of data, Hydrochloric Acid concentration vs Time.
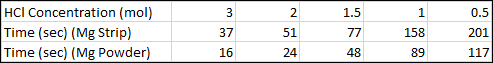
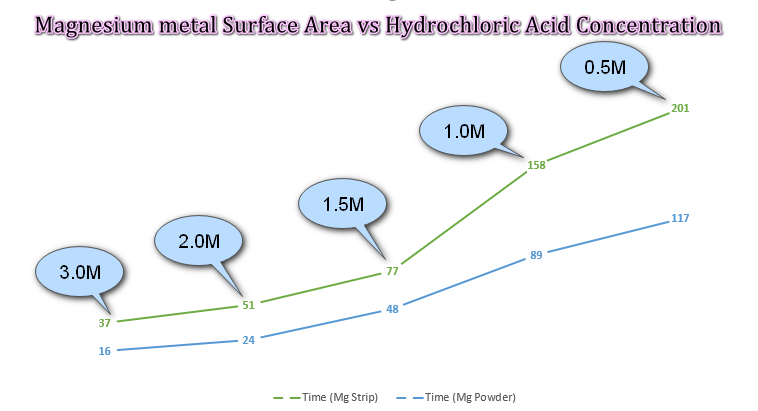
(b) Give an explanation of your interpretation of the data, does there appear to be any correlation between surface area and rate of reaction?
It can be seen from the data that in both cases, using solid magnesium strip or magnesium powder the time taken (in seconds, as shown on each data point) for the reaction to complete is inversely proportional to the concentration of the acid used. In other words, as the concentration of the acid increases, the time taken for the reaction to complete decreases. Both "solid" and "powder" show a similar correlation, but in the case of the powder it can be seen that the reaction is far more rapid, ending in approximately half the time per data point. This is consistent with the fact that powdered magnesium will have a greater surface area and therefore the probability/frequency of successful reaction "collisions" is increased.
Q2. In the "downward displacement of water" method of measuring the reaction rate of this particular reaction, it is important that the amount of hydrogen gas produced does not exceed the capacity of the measuring cylinder used to capture it. If this was allowed to be the case, there would be no way to accurately measure the data once the measuring cylinders volume had been exceeded.
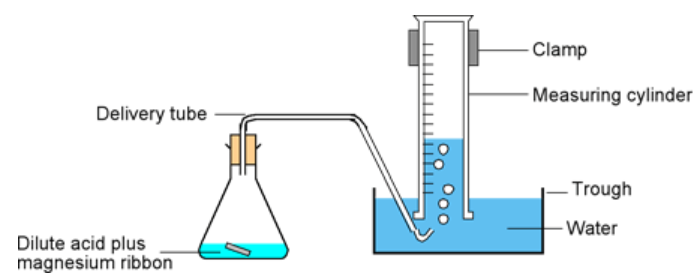
Explain how this experimental error could be avoided with consideration to how much Magnesium metal is actually used.
This isn't a particularly hard question, it relies on your knowledge of the volume at RTP of 1 mole of a gas, knowledge of the balanced chemical equation for the reaction involved and a little bit of mathematics to calculate the amount of Magnesium that should be considered.
The reaction proceeds according to the following equation:
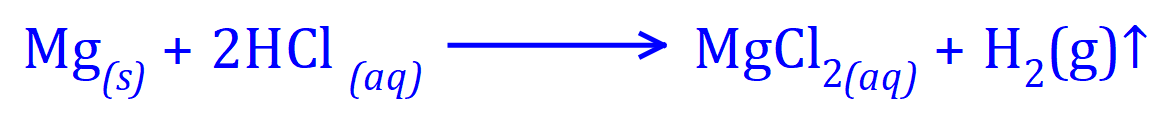
Your understanding of moles and molarity should tell you that the reaction is one-to-one with respect to Magnesium and Hydrogen gas molecules produced.
"One mole of Magnesium reacts with two moles of Hydrochloric Acid to produce one mole of aqueous
Magnesium Chloride solution and one mole of Hydrogen (molecular) gas"
If we were to use one mole of Magnesium metal, 24.3 g we would produce one mole of diatomic Hydrogen, which would have a mass of 2.016 g and a volume (at RTP) of 24 dm3. It is the volume of gas produced that we are interested in and this is the factor which we need to control. If we were to be using a 100 mL/cm3 measuring cylinder we would need to make sure that the amount of gas produced did not exceed this value.
Given the fact that 1 mole of Hydrogen gas occupies 24,000 cm³, (24 dm³) we can calculate a "working" value of gas which we need to produce, and from this calculate the correct mass of Magnesium metal. Let us say that we wish to produce approximately 80 cm³ of gas so that our experiment is not ruined by overproduction, we could work out how many moles this is quite simply:
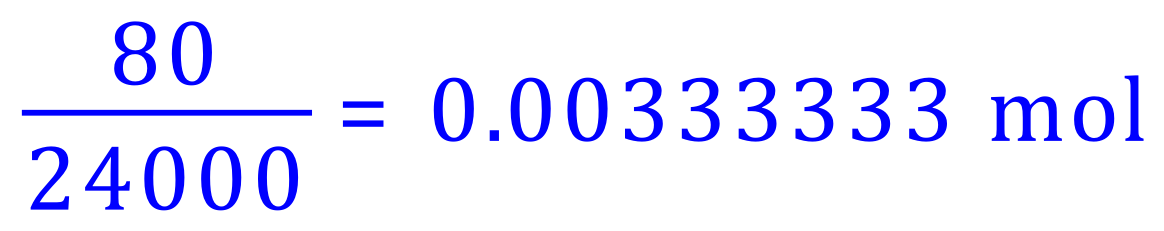
We have seen previously that the reaction is one-to-one with respect to Magnesium and Hydrogen, therefore to produce this many moles of Hydrogen gas would require us to use the same number of moles of Magnesium. It is a simple matter of arithmetic to work this out as a mass in grams:

A 2 decimal place laboratory balance would allow us to measure out 0.081 g (81 mg) of Magnesium.
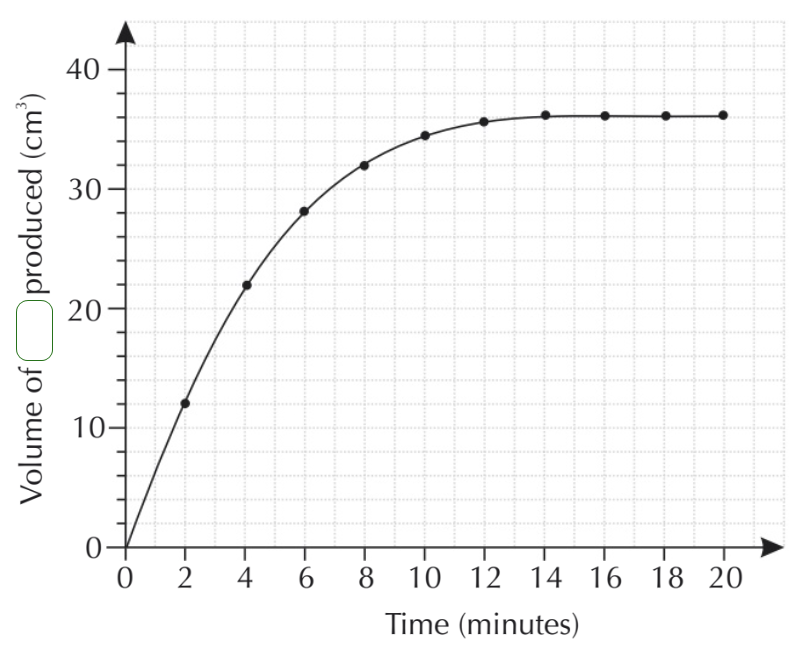
Q3. A student conducted an experiment using Hydrochloric Acid and Calcium Carbonate to study the rate of reaction by evolution of a certain gas. The data was collected and from this a graph produced:
(a) Name the gas produced in this reaction, and show the balanced chemical reaction with state symbols:
The gas produced is Carbon Dioxide CO2, produced according to the following equation:

(b) From the graph, state how long it took for the reaction to complete.
The reaction was complete by 14 minutes as this is where the curve 'levels out'
(c) What was the rate of reaction, in cm3 per minute on the third reading?
At reading number 3, the rate of reaction was 28/6 or 4.67 cm3 per minute.
(d) Give a brief explanation as to why the reaction slows down as time progresses.
As the reaction continues, more a product is being made and more and more reactant is being used up. The number of successful collisions between reactant particles is reducing as the availability of reactant drops, also there will be collisions between reactant particles and product particles (which are on the increase) and this will have the effect of slowing down the rate of successful collisions between reactant particles.
(e) The student repeats the experiment by keeping the mass of Calcium Carbonate constant, but reduces the concentration of the Hydrochloric Acid by half although it remains in excess. Another curve is plotted and the same axes are used, the student notices that although the curve is not a sharp the first one the same amount of carbon dioxide is produced as the curve levels out at the same place (the curves overlap). Explain why this is?
The clue here is the fact that the question states that Hydrochloric Acid will be in excess, this is to make sure that all of the Calcium Carbonate is used up although because the acid is weaker, the number of collisions between react and particles will be reduced per unit time, in other words the reaction will proceed much more slowly. However, the balanced chemical equation still stands, and as the same amount of Calcium Carbonate has been used, the same amount of Carbon Dioxide will be produced although over a longer period.
Back To >> Questions <<
Back To >> Measuring Rate Of Reaction <<